The sciatic nerve is a large nerve that comes from the
lumbosacral plexus. The sciatic nerve has five nerve roots, L4, L5, S1, S2, and
S3. The sciatic nerve runs from the lower spine, through the buttock to the
lower leg and foot. The sciatic nerve initially emerges from the pelvis and
exits the greater sciatic notch anteriorly and deep to the piriformis muscle,
exiting below the piriformis muscle.
The sciatic nerve then enters the thigh
between the ischial tuberosity and the greater trochanter of the femur. In
about 10% of patients, the sciatic nerve is separated by all or part of the
piriformis muscle. The sciatic nerve enters the thigh beneath the lower border
of the gluteus maximus muscle. The nerve then runs down and branches out within
the posterior aspect of the thigh, down to the leg and foot. The sciatic nerve
gives multiple sensory and motor branches to specific areas and muscles in the
leg and foot. The two main branches of the sciatic nerve are the posterior tibial
nerve and the common peroneal nerve.
Irritation of the sciatic nerve may occur at multiple sites.
The first site that we need to look at is the spine—which is where irritation
may occur, usually from lumbar disc herniation. This is considered true
sciatica or lumbar radiculopathy. Another site for irritation of the sciatic
nerve is at the piriformis muscle. The sciatic nerve may become compressed by
the piriformis muscle (piriformis syndrome). Piriformis Syndrome is a diagnosis
of exclusion! If the patient has symptoms of sciatica, then there must be an
MRI of the spine that is negative, proving that the symptoms are not associated
with a possible disc problem. Once the MRI is negative, then you can say that
the condition of sciatica may come from piriformis syndrome.
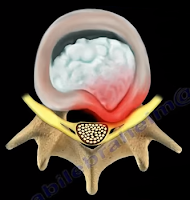 |
| Disc Herniation |